Project Description
Author: Hasan et al.
Summary:
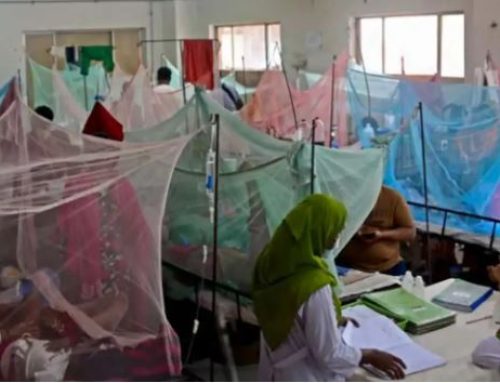
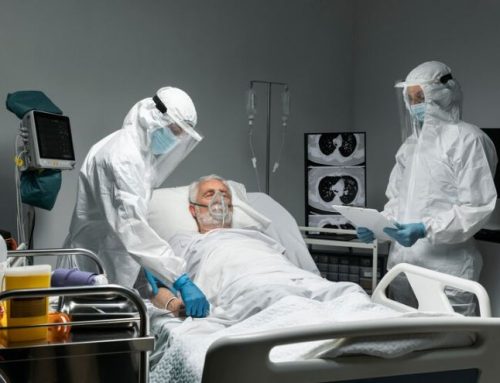
Dengue is responsible for significant morbidity and mortality around the globe. Current recommended treatment is largely supportive with careful fluid replacement and associated care. Existing data do not recommend routine use of steroids in the management of dengue fever and are not mentioned in the WHO or national guidelines on the management of dengue. However, corticosteroids are used empirically based on the presumed immunological basis of the complications of dengue. The evidence base for the benefit or lack of benefit of corticosteroids in dengue is limited. Previous studies are small with methodological flaws eg. less stringent randomization, and were performed a long time ago and mostly in the pediatric group. Therefore, the aim of this study is to assess the efficacy of methylprednisolone in management of patient with decompensated dengue shock syndrome with or without expanded dengue syndrome. This phase III double blind randomized controlled trial will be conducted at the department of medicine in DMCH, for 6 months of duration approximate September 2019 to February 2020, following approval of this protocol. Adult patients (age>18 years) with diagnosed dengue and in decompensated shock with or without expanded dengue syndrome will be selected for this study. Written informed consent will be taken from the participants. In case of low level of conspicuousness, consent will be ensured from the attendants of their patients. Total 362 patients will be selected for this study and will be randomly assigned into two groups (Group M and P) with a ratio of 1:1. Each group, 181 patients will be included. Group P (Placebo) will receive standard treatment protocol according to National guideline + Placebo and Group M (Methylprednisolone) will receive Methylprednisolone 1gm intravenously for 3 days in addition to standard care. All participants and study physician (and Nurses) will be blinded to the treatment allocation. Patients’ vital sign will be closely monitored. Primary outcome will be to measure mortality rate and secondary outcome will be to measure any adverse events, duration of shock, hemodynamic stability, need for ICU admission, lowest platelet count, percentage increase of the maximum hematocrit from baseline, and methylprednisolone related side effects. The data will be systematically described and summarized and presented through descriptive statistics and final will be analyzed by the statistical program Statistical Package for Social Science (SPSS) version 23.0 (Chicago, Illinois, USA). Relevant statistical test will be used during analysis. In all cases significance level will set p< 05.
Keywords: ……………….
Status: Ongoing
Full text link: Not available